-
Larval Food Host
-
The mistletoe Amyema miquelii (box mistletoe) (Loranthaceae).
This mistletoe normally only associates with Eucalyptus. The larvae eat the
flower buds, flowers, leaves and soft stem parts of the mistletoe, but are particularly
fond of the flower buds.
-
Larval Attendant Ant
-
In southern areas of the butterfly's range in South Australia, larvae are
usually attended by a few small black or dark brown and black ants. Fat
abdomened Crematogaster species are the usual
ants in attendance, although on northeast Eyre Peninsula
Doleromyrma and Iridomyrmex
species are usually in attendance, and in one case the black stinging
Rhytidoponera metallica was present.
In the Ooldea area within the northern area of the butterfly's known range
in South Australia, small black Iridomyrmex ants
have been seen on the host plant harbouring the early stages, but more often
in this area, ants are not present.
In the eastern states, early stages are also known to be attended by
Anonychomyrma sp, Camponotus sp,Froggattella kirbii,
Meranoplus sp, Ochetellus sp, Podomyrma sp and
Tetraponera sp.
-
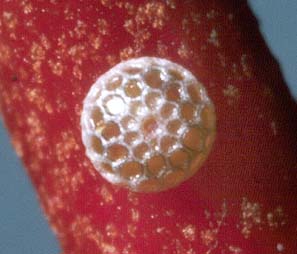
Eggs
-
Small, greyish white with areas of dark grey, hemispherical shape, basally flattened,
the top is domed. The sides are coarsely reticulated in a hexagonal pattern that
continues to the edge of the small pale coloured, depressed micropylar area at the
apex of the egg. At each reticulation intersection there is a small raised blunt
projection. The egg is typical of the eggs for the O. olane Species Subgroup.
They are normally laid singly, usually on the stems and flowers buds of the mistletoe,
but also on the leaves and bole of the mistletoe. There is often a very large population
of tiny parasitoid wasps on the mistletoe and very few eggs survive parasitisation.
-
Larvae
-
The first instar is pale greyish yellow, later becoming pale green after eating the
young leaves of the hostplant. Long onisciform shape, with scalloped lateral edges.
The posterior dorso-lateral organs are not developed. The head is large, smooth,
brownish yellow, hidden beneath the body. There are long dark peripheral and dorsal
setae, which are longest anteriorly, posteriorly and dorsally. Dorsal setae occur
in two pairs, one pair being long and recurved, the other pair being short,
recumbent and directed to the rear. Intermediate instars gradually lose the long
dorsal setae and gain the posterior dorso-lateral organs, and are onisciform
(slater shaped).
First instar larvae remain exposed on the leaves of the mistletoe. Subsequent instars
usually hide during the day, and feed at night, sheltering during the day beneath
bark at the base of the mistletoe or on the adjacent host tree, or in crevices in
the mistletoe host, or in borer holes or ant tunnels in the mistletoe or mistletoe
host, but always as near as possible to the mistletoe. If there is no bark near the
mistletoe, then they will shelter beneath the nearest available bark below the
mistletoe on the host tree. However, pale coloured larvae may remain exposed on the
hostplant, although quite often these larvae are parasitized. The young larvae
initially eat the young tips of the mistletoe, but quickly graduate to older leaves
and flower buds. There are usually only one or two larvae present on each mistletoe
that are being utilised by the butterflies, and very few mistletoes actually get used.
The fifth (final) instar is about 22-24 mm long, polymorphic being either some shade of
brown, brownish pink or yellowish green. The brown larvae are marked similarly to other
small Ogyris in South Australia, with pale yellow dorsal chevron markings, and
other subdorsal markings, and the anterior and posterior extremities are usually free
of pale markings. In the pink and green larvae the markings are indistinct. Onisciform
shaped with a thoracic dorsal furrow, the lateral edges are scalloped, and the anterior
and posterior areas are flattened, and there are some short peripheral hairs that are
longer anteriorly and posteriorly. The body is covered in pale and dark coloured, minute
secondary setae, which are recumbent, club shaped and spiny in appearance, set on a
protuberant,angular and ridged base. The secondary setae impart a scabrous appearance to
the larvae. The posterior dorso-lateral organs are well developed. The head is small,
smooth, yellow, hidden beneath the body.
In the Flinders Ranges and Riverland areas, larvae have been known to occur with larvae of
O. amaryllis.
-
Pupae
-
Short cylindrical, rounded anteriorly and posteriorly, about 13-15 mm long, brown
coloured with other darker cryptic markings, and there is a darker dorsal line and
usually a single row of dark subdorsal dots. There is usually a pair of large, dark
brownish dorsal spots above the head. The surface of the pupa is without hairs, but
bears a very fine reticulated pattern (similar to the eggs) that produces a very fine
scabrous surface, and which includes tiny specialised secondary setae that are more
common around the spiracles. The pupa period is about 24-27 days in late spring.
Pupation usually occurs in the first lot of secure bark on the host Eucalyptus,
below the mistletoe, often in the first crook of the tree below the mistletoe.
Occasionally they will pupate in the final larva shelter near the mistletoe, or near
the base of the host Eucalyptus well away from the mistletoe. Larvae usually
pupate singly, but will also pupate next to O. amaryllis if they are also on
the same tree. Pupating larvae surround themselves with a loosely webbed, silken
cocoon-like shelter, which presumably affords some protection from larger predators,
or perhaps from other Ogyris larvae which can cannibalise soft pupating larvae.
There is normally space within the silk webbing for the small attendant ants to pass
through to attend to the live pupae. Pupae are attached to the silked substrate by
anal hooks and a central girdle.
The pupae are known to stridulate, making a series of audible clicks, which are believed
used as a means of communicating with the attendant ants. There are normally several ants
in attendance with a pupa, but sometimes there are none.
-
Flight Period in South Australia
-
The butterfly flies throughout the warmer months, with records from September to early June,
and there is a single captivity record in early July.

-
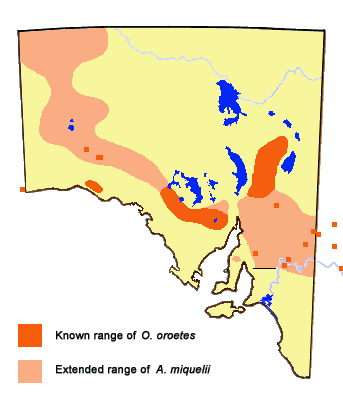
Distribution
-
In South Australia the butterfly only exists where its mistletoe hostplant occurs.
It is essentially a tropical-subtropical to hot temperate butterfly and is unable
to tolerate continuous cold temperatures, and is probably limited to average annual
minimum temperatures above about 10-11°C. The butterfly occurs mostly in the pastoral
areas where very little survey work has occurred, and based on the known distribution
of its hostplant in the Northwest Region of the state, the butterfly is likely to have
a more extensive distribution than is presently known. The distribution of hostplant in
this area is very localized and discontinuous. There are also isolated occurrences of
the hostplant in the central and northeast areas of the Far North Region of the state,
but are probably not of high enough density to support viable colonies of the butterfly.
There is a recently confirmed record of the butterfly occurring at Clare in the North Mt
Lofty Range of the Midnorth Region, but the elevated North Mt Lofty and Southern Flinders
Ranges would normally be precluded as potential habitat due to the likelihood that most
of these areas would be too cold, even though the hostplant of the butterfly is present.
However, with the recent elevation of temperatures due to drought and 'climate change'
since about year 2000, the range of this butterfly could be creeping southwards. From
recent studies in WA there is a belief that the butterfly may be able to marginally
extend its southerly flight range into cool temperate areas by having a flight during
the hot autumn months and producing eggs that diapause during winter-spring.
Similar to its relative Ogyris amaryllis the butterfly
tends to form a phenotypic (environmental) cline across Australia with respect to morphology
and consequently the local population has attributes somewhere between the populations in
eastern Australia (nominotypical subspecies), populations in southwest West Australia
(subspecies apiculata) and the Central and North-west Australia populations (arid form).
The local population is presently included under subspecies apiculata although there
is no sound reason for this.
-
Habitat
-
In SA the butterfly occurs in hot temperate and semi-arid pastoral open mallee woodland.
-
Conservation Status in South Australia
-
The butterfly is very rare in its occurrence, but widely distributed.
-
Threats
-
Not generally under threat except where the habitat is adjacent to farming communities and
affected by poison spray drift during crop spraying activities, especially when the poison
is applied by aerial means. Parts of its eastern range are within the prime breeding grounds
for the plague locust
and the butterfly would be decimated by any toxic spray programs adopted by the Locust Control
Board (as happened in year 2000).
-
Conservation Strategy
-
None required, except that any spraying activity should be carried out judiciously.